Integrated Life Systems (ILS)
Innovations Transforming Biotechnology and Healthcare
Discover ILS
ILS introduced cutting-edge innovations in biotechnology and healthcare. Our biotechnology and medical devices are revolutionizing product development cycles for sectors like diabetes, cell biology research, molecular diagnostics, and biotech and pharmaceutical manufacturing.
ILS efforts are focused on providing first/best in-class solutions that address significant challenges facing researchers, manufacturers, care providers, and patients. Our purpose is to offer useful tools to facilitate efficient pharmaceutical production, leading to safe and affordable healthcare.


Our Innovations
Integrated Life Systems introduced transformative innovations advancing the biotech and healthcare industries. The technologies provide qualitative and quantitative advantages in sectors such as diabetes, cell biology research, molecular diagnostics, and biotech manufacturing. ILS devices benefit patients, medical professionals, biotech and pharmaceutical makers.




Non-Invasive Glucose Detection
Groundbreaking Advance: ILS introduced a unique Non-Invasive (NI) Detection technology. ILS approaches NI challenges from a completely novel view that ILS believes would be of great benefit to healthcare professionals and diabetic patients; effectively eliminating the need for blood draw and needle use, and facilitate convenient glucose monitoring.
Impact on Diabetics: According to the American Diabetes Association, there are currently over 37 million Americans living with diabetes, accounting for 11.3% of the population, and an additional 96 million individuals that may be identified as pre-diabetics . ILS technology would provide a fast, comfortable and completely pain free means to measure glucose blood levels, which increases compliance with medication regiments, eventually leading to improving life quality and reducing health care.

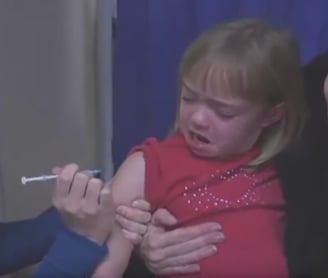
Note: Although continuous glucose monitors (CGM) reduce the frequency of skin puncture, they require the use of reagent patches and a probe (needle) that must be inserted into the patient's body, and be regularly replaced. In contrast, ILS devices do not require needles or reagent patches.
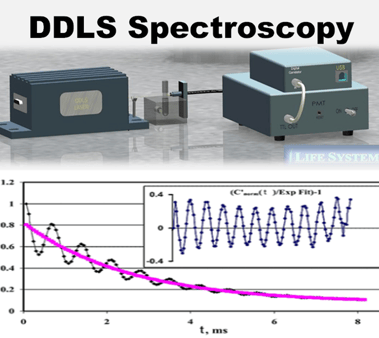

ILS has been granted patents for the DDLS and Non-Invasive detection
Benefits and Applications
DDLS Spectroscopy creates a New Platform for development of novel medical and analytical instruments
Bacterial and viral identification
For biological cells, infection, mutation, or environmental assaults changes cell properties. DDLS measurements detects the changes, useful applications in, e.g.:
in-vitro Cancer Cells ID
Circulating Tumor Cells (CTC)
QA of sensitive biological fluids without the need to open or sample the fluids, e.g., monitoring blood bags viability
DDLS measurements are fast (Seconds to Minutes)- compared to hours in, e.g., FISH (Fluorescence in-situ hybridization)
No Reagents (Specific to DDLS) Required
No requirement to “fix” or kill cells
Greater resolving power in characterizing complex heterogeneous populations, useful for identification of complex solution: inorganic and organic polymers mixtures, e.g., industrial polymers, DNA, RNA, etc.
ILS's NI technology is based on a novel spectroscopy, the Dielectrophoretic Dynamic Light Scattering (DDLS) spectroscopy, introduced by ILS. DDLS has a wide-range of applications in particle and cell detection, from latex particles characterization to cancer cell diagnostics.
Briefly: DDLS incorporates multiple scientific and technological disciplines. DDLS measurements are carried out in an oscillating electric field, which induces macromolecules to undergo characteristic motion. The motion is detected by the modulation in laser scattering (time autocorrelation function). Such motion is characteristic of the macromolecular biophysical properties, e.g., Size, Electrical charges, shape, etc. The properties are indicative of (and change with) cell conditions, e.g., metabolism, sugar level, growth state, infection by microbes, etc.
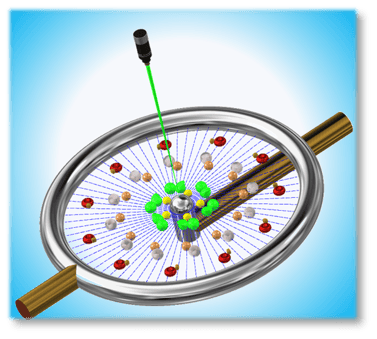
A Unique cell breakage (lysis) device. Lysis breaks cell membranes to release genetic and other interior cell materials of interest to researchers in general, medical, biotech, and other fields.
Lysis is a prerequisite for many biotech operations and diagnostics testing, e.g. PCR. The Lysor therefore addresses a significant market applications.
The Lysor was used in clinical and market units, with FDA (510k) approval
The ILS Lysor®
The ILS Lysor technology is based on the creation of standing ultrasonic plate waves. At particular geometries and frequencies, maximal/minimal energy “circles” are formed.
Sample Containers (usually closed tubes) are laced at Maximal Energy locations, which enhances lysis efficiency. The Lysor was used to release nucleic acids from Mycobaterium Tuberclosis (MTb), as a critical components in an MTB PCR assay..
Shown to efficiently lyse Mycobacterium tuberculosis (MTb) in few seconds.
Advantages of the ILS Lysor
The Lysor development presents a first, best-in-class device for cell lysis to release/extract desired cell components.
The Lysor advantages include: High Throughput as more sample tubes can be utilized with larger plates; No Cross Contamination with proprietary ILS sample tubes; Unique consumable, ILS sample vessel, ensures maximal transfer of vibrational energy to sample; and, Operator Safety, as no escaped aerosols.
ILS has been granted multiple patents for its non-invasive devices.




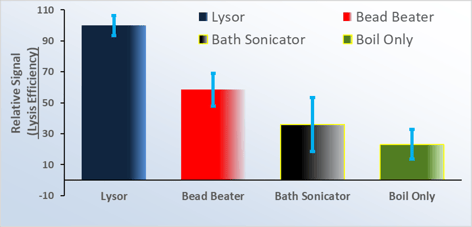
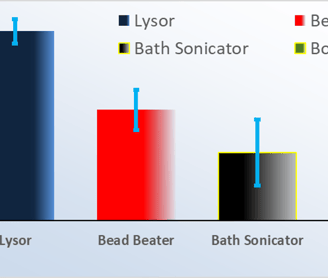
Superior Performance
The Lysor efficiently and quickly* lysed ‘tough’ microorganisms (e.g., Mycobacterium Tuberculosis, MTb); outperforming competitive lysis methods.
Bio Purification
Importance
Bio Purification is crucial
Manufacturing of pharmaceuticals, biotechnical, chemical, and food industries necessarily require purification to assure compounds' safety and efficacy. Purification can add substantial cost to delivering, e.g., a purified monoclonal antibody (MAb) drug, often constituting more than 70% of total manufacturing cost.
Critical Industries and Vast Markets Biopurification serves the pharmaceutical and biotech sectors which are pillars of the international economies with large sophisticated customer base.


Status Quo Challenges
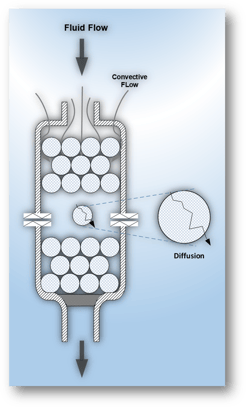
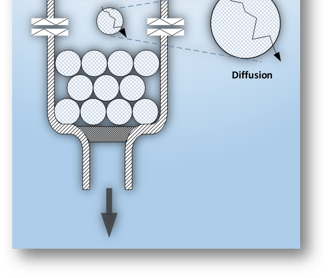
Purification Products can be generally categorized as polymeric particles, e.g., gel beads, and membrane-based, e.g., hollow fibers.
Gel beads are widely used, they may have large capacity but exhibit poor flow characteristics. The major share of their capacity is reached by Diffusive Flow. Diffusion rates are significantly slow compared to Convective Flow. Large bead columns require high pressure to achieve appreciable flow which may deform delicate proteins and the beads themselves. Column packing and support require substantial time and skilled personnel.
Membrane‑based products, e.g., hollow fibers and flat sheets may be fast but their lower capacity limits scale up.
Solution: ILS SmartGel©
SmartGel Columns Applications
ILS has produced a variety of SmartGel purification columns to address extraction of a variety of classes of molecular species.
Columns with functionalities of ion exchangers, nucleic acids, affinity (antibody/antigen), heavy metal chelation, and hydrophilic filters have been prepared and utilized.
The columns demonstrated remarkable bind and release performance of compounds including immunoglobulins and other proteins, DNA/RNA, and multivalent ions. SmartGel were shown to exhibit outstanding performance compared to on-market competitors.
SmartGel columns are provided in capacities from milligrams to large scales up to kilograms. This facilitates migration and transferability from R&D to larger scale production, with substantial reduction cost and in process development time.
An exciting prospect of SmartGel technology is to address the extraction and recycling of heavy metals and other environmental hazards.
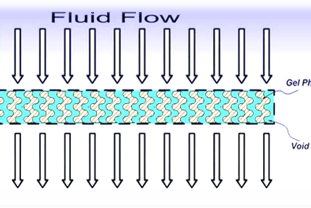
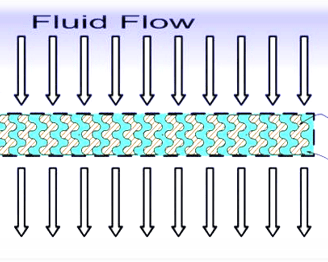
ILS introduced SmartGel, a novel purification matrix that creates an efficient "net" to specifically capture target molecules in complex fluids. The matrix is tailored to efficiently captures and release the target molecules.
SmartGel matrices are formed in various devices configured to operate in substantially convective flow. Combined with high capacity, fast convective flow enables SmartGel devices to rapidly extract and concentrate molecules from very dilute/large volumes.

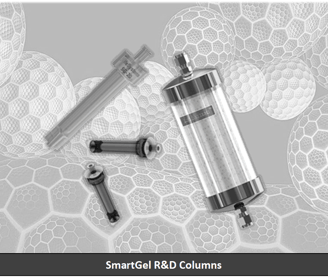
Major Improvements for Large-Scale Purification:The challenges with large scale for the now routine and life saving biopharma drugs can be addressed and overcome using SmartGel, with high capacity and significantly higher fluid flow rates at significantly low pressure. This enables purifying delicate protein drugs and bio-pharmaceuticals while avoiding degradation of delicate biomolecules.
Flexibility: A wide range of SmartGel products are manufactured using essentially the same process, with capacity from milligrams quantities(R&D) to industrial scale. This eases migration from R&D to larger scale, substantially reduce process cycle duration.
Wide Application Fields: Proteins, Nucleic acids (DNA & RNA); Heavy metal ions, Filters, etc.
Ease of Use: The availability of plug-in SmartGel columns provides a substantial market advantage over bulk gel particles due to time savings in column preparation and validation.
Lower Consumables, Labor, and Operating Cost: Columns are provided in ready-to-use or customizable formats; lower capex; and smaller foot print. SmartGel columns also enjoy a strong margin advantage versus poured gel beads.
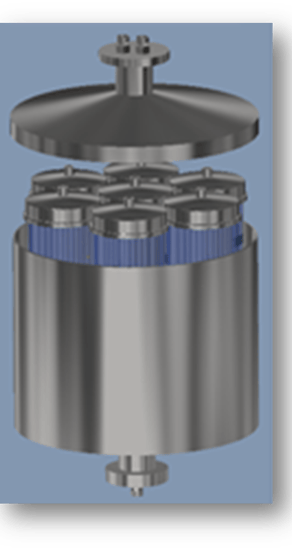

SmartGel Columns: Extraordinary Advantages
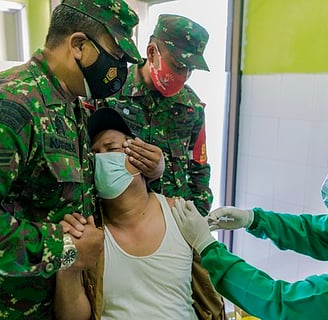
Nerve endings in skin layers are sensitive to medical treatments that penetrate/interact with the skin. Pain may be severe enough to require administration of pain relief drugs, such as Novocain. Avoiding pain relieving drugs is preferable, whenever possible.
Importantly, the associated pain levels can cause patient apprehension, discomfort, and may interfere with medication compliance. This is relevant in case diabetics, and particularly diabetic children.
Skin Cooling Devices



Cooling of the skin has been observed to lessen pain associated with hypodermic injections. Skin cooling can improve patient experience during hypodermic injections.
Cooling skin is attempted by methods including evaporative anesthetics (vapocoolant), where a volatile anesthetic is sprayed on the area of injection (Link). Another method uses a distraction device (for children) with a buzzing sound.
Other methods include use of refrigerated needles (needles stored in a freezer), use of cooling gas, and use of chemical (endothermic) reactions, e.g. ammonium nitrate and water (Link).
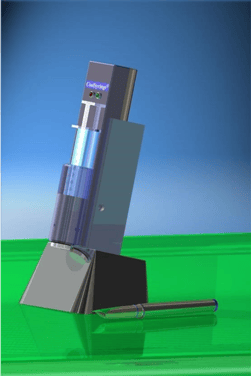
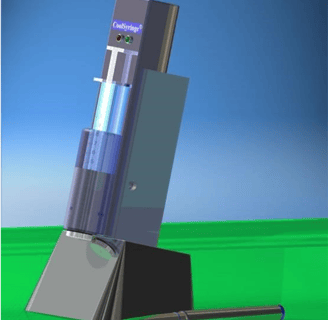



CoolSyringe Help Reduce Pain in Many Procedures
Fear of Needle: Needle Phobia is not exhibited by children only; over 20% of adults may temporarily lose consciousness around the time of a needle procedure (Wikipedia).
Diabetes: 37.3 million people, or 11.3% of the U.S. (Link). population, have diabetes. An estimated 28.7 million people – had diagnosed diabetes. Approximately 8.6 million people have diabetes but have not yet been diagnosed. In addition to needle apprehension, frequent injections will likely irritate/cause irritation and multiple skin bumps.
Cosmetics: Filler injections; e.g., Botox/Others. Note that Botox is also prescribed for migraines. Numerous other injectable medications/cosmetics routinely enter the market, e.g.,:
Childhood Vaccines: 26 vaccine shots are given per child in child's first year currently in the USA, where infant/other childhood vaccine shots are estimated at >100 million per year.
Seasonal Vaccination: Annual flu shots are administered to about 50% of the population; >100 million injections/year.
COVID-19: By the end of 2022, multiple billions of COVID-19 doses have been administered worldwide; 600 million in the USA.
CoolSyringe: Easy, Cool Pain Relief
CoolSyringe offers advantages currently unavailable in commercial or clinical devices that address pain reduction via skin cooling. This may be concluded from the competition outlined above, and after patent and literature searches.
CoolSyringe’s advantages include
Solid State: no fluids, chemical reactions, or evaporative anesthetics;
Easy of Use: Hand-held; Delivered Ready to use;
Battery-Operated, Rechargeable;
Fast skin cooling (seconds);
Safety Features include low voltage operation, temperature monitoring indicators, automatic shutoff.
ILS has been granted multiple patents for its skin cooling devices.
CoolLaser alleviates pain during dermatological procedures involving electromagnetic sources, e.g., lasers. CoolLaser’s applications include procedures such as hair and tattoo removal, skin de-pigmentation and other dermatological cosmetic procedures. These are widely used applications, for example, there are over 1 million hair removal procedures yearly in the USA.
Currently available laser devices used in cosmetic treatments with cooling options, particularly in professional medical settings, are quite large, use liquid or gas for cooling, and require professional maintenance (Link).
Note: ILS does not address manufacturing the lasers or other sources of electromagnetic radiation. ILS niche is to provide simple, portable and elegant cooling implements for patient comfort and pain reduction during cosmetic procedures.
CoolLaser offers advantages to afford its use by medical professional and home users with features including: Solid state construction; fast skin cooling, hand-held small form factor, battery-operation, no fluids or cold gases used, and use safety.
For more information on CoolLaser, please contact ILS.








